A new treatment target for Fragile X syndrome has emerged from multiple research labs and a pharmaceutical startup. Neurolixis, a FRAXA pharma partner, has announced a new Fragile X development program.
Read moreAuthor: Michael Tranfaglia, MD
BK Channel Openers: A New Drug for Fragile X Is Ready for Clinical Trials
Discover the promising new BK channel opener, SPG601, now entering clinical trials for Fragile X syndrome. Learn about its potential to restore synaptic function and address core symptoms.
Read morePioneering Community-Based Drug Development for Fragile X Syndrome

Discover how FRAXA leverages Community-Based Drug Development to create impactful therapies for Fragile X syndrome. Join us as we reshape the future of rare disease treatment.
Read moreInnovative Breakthrough in Fragile X Treatment: The Promise of Antisense Oligonucleotide (ASO) Therapy

This changes everything! FRAXA funded research introduces Antisense Oligonucleotide (ASO) Therapy, redefining Fragile X syndrome treatment and understanding.
Read moreUnraveling Fragile X Syndrome: New Insights into FMR1 Gene Reactivation

Discover groundbreaking methods for reactivating the FMR1 gene in Fragile X syndrome. Dive into the transformational research and the implications of self-healing at a cellular level.
Read moreBreakthrough Discoveries in Fragile X Research: Insights from Special Banbury Meeting on Curative Therapies

Explore the latest breakthroughs in Fragile X research unveiled at the recent Banbury Meeting. Discover novel strategies, from gene therapy to protein replacement, that bring hope for curative therapies.
Read moreFragile X Syndrome and Cancer Research: Unexpected Links and Opportunities for Collaboration

Discover unexpected links between Fragile X Syndrome and cancer. Studies show people with Fragile X have much lower cancer rates. Explore new opportunities for collaboration in this promising research.
Read moreConsidering Available Drugs for Fragile X: My Favorite Combination (So Far)

Which of the available drugs are best for fragile X? We tend to think of drugs according to their primary activity in the body, but very few drugs are totally selective and specific. There are differences between drugs in any given class, and these differences may be critical. Most drugs have “off-target” effects which are usually considered side effects, and it is these side effects which can have key advantages, in some cases.
Read moreRepurposing Available Drugs to Treat Fragile X Syndrome – FRAXA Initiatives
FRAXA Research Foundation was founded in 1994 to fund biomedical research aimed at finding a cure for Fragile X syndrome and, ultimately, autism. We prioritize translational research with the potential to lead to improved treatments for Fragile X in the near term. Our early efforts involved supporting a great deal of basic neuroscience to understand the cause of Fragile X. By 1996, these efforts had already begun to yield results useful for drug repurposing. To date, FRAXA has funded well over $25 million in research, with over $3 million of that for repurposing existing drugs for Fragile X.
Read moreCRISPR – Does it hold promise for Treatment of Fragile X Syndrome?
There’s been a lot of press concerning a new biotechnology called CRISPR/Cas9, or simply CRISPR. This technology, which is based on the discovery of naturally-occurring bacterial defense mechanisms, has attracted an enormous amount of biotech investment. It has also excited the imaginations of scientists, clinicians, and rare disease advocates everywhere. How might CRISPR be applied to Fragile X syndrome? CRISPR offers the tantalizing possibility of “editing” genes very precisely, and it could (theoretically) excise the methylated trinucleotide repeat sequence from Fragile X cells, rendering them entirely normal.
Read moreFRAXADev – Developing BK Channel Openers for Fragile X Syndrome

A number of people have asked us about FRAXADev, a new project starting in France; this is a nonprofit initiative which seeks to develop a new kind of drug for Fragile X. The drugs they are interested in testing in Fragile X clinical trials were developed by Bristol-Myers Squibb many years ago, and are now off patent. This class of drugs opens a potassium channel in the membrane of neurons, which helps to decrease neuronal excitability.
Read moreFragile X: Past, Present, Future – Video

A Fragile X presentation was given by Michael Tranfaglia, MD, FRAXA Medical Director at the IDD-C conference at Stanford University on April 21, 2015. This was an in-depth discussion of how research has brought us to the point of clinical trials, the problems encountered in recent trials, and where we go from here. Dr. Tranfaglia presents new ideas on Fragile X disease mechanisms and new treatment strategies which may address these.
Read moreFragile X Treatment: New Research Directions
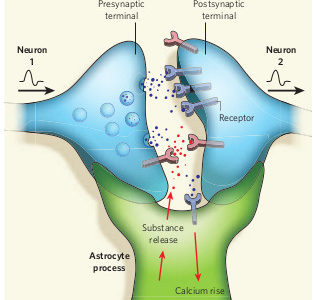
In the wake of negative results from several high-profile clinical trials in Fragile X, we find ourselves questioning many of our previous assumptions about the nature of this disorder. After all, understanding the basic pathology of disease is critical to development of new treatments — this is true across the board, in all branches of medicine.
Read moreNeuren’s NNZ-2566 Shows Clinical Benefit in Rett Syndrome Trial
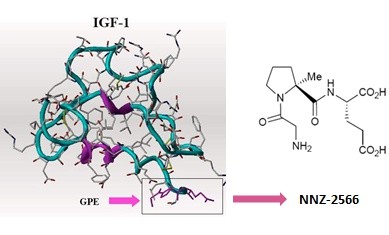
This isn’t a Fragile X trial, but the Neuren compound, NNZ-2566, that is in trials now for Fragile X has shown significant positive effects in a Phase 2 trial for Rett syndrome. The results of the trial are interesting, in that improvement was seen a Rett syndrome-specific rating scale compared to placebo, and there was also improvement noted on the CGI-I (Clinical Global Impression of Improvement) and Caregiver Top 3 Concerns. However, there was no effect seen on ABC scores (Aberrant Behavior Checklist) compared to placebo. Many in the Fragile X field have noted the inadequacies of the ABC; indeed, it was never designed or intended to be an outcome measure for clinical trials.
Read moreRoche reports clinical trial negative results

Roche has shared the sad news that their clinical trials in Fragile X have been unsuccessful. They will host a Webcast on Thursday, September 18, from 12:30pm – 1:30pm (EDT) to explain the results. For details and dial-in information please see this letter from Luca Santarelli, the
Read moreWhy Did Fragile X Clinical Trials of mGluR Antagonists Fail?

by Michael Tranfaglia, MD. In my opinion, the Fragile X clinical trials of AFQ056 sponsored by Novartis failed because of a dose range that was inadequate for Fragile X, and because of the unexpected development of tolerance.
Read moreFragile X Syndrome Treatment Target: MMP-9

Dr. Ethell was awarded FRAXA Research Foundation funding from 2008-2011 and 2012-present. This latest work shows that human Fragile X tissues have elevated levels of the extracellular enzyme MMP-9, as well as an increase in the active fraction of that protein (like most enzymes, MMP-9 can exist in an inactive form which can be switched on rapidly; this kind of regulation is important in most biological pathways.)
Read moreFragile X Clinical Trial: Novartis Trial Results Are In, and They’re Not Pretty
This year’s Gordon Conference just finished, and Novartis presented their results for the first time (though advisors and advocates had been given a private peak months ago.) To say that the trial results for AFQ056 were disappointing would be the understatement of the century!
Read moreWhat Treatments Work for FXTAS?
Many older family members in the Fragile X community are affected by FXTAS (Fragile X-associated Tremor/Ataxia Syndrome). We all hope that knowing the underlying cause of neurodegenerative symptoms in FXTAS will help in the development of specific treatments over the long term. In the short term, we would also hope that having a specific diagnosis would help us to identify particular available treatments which might be more effective than others.
Read moreFRAXA Progress and Future Plans
It’s official: Fragile X is now the hottest research topic in all of neuroscience! Just last month, massive publicity attended the publication of Fragile X clinical trial results and major papers by FRAXA’s outstanding translational researchers. Even as news of the first round of Fragile X clinical trials is emerging, Phase III trials (which can lead to actual marketing of medications) are under way and slated to finish around the end of the year. This is exciting, and it is just the tip of the iceberg.
Read moreWhat Works, and What Doesn’t
At the start, it’s always hard to know what methods will work best for something as complex as the development of disease-modifying treatments for Fragile X. But, we’ve always tried to let the science lead us down the right path. At this point, the results are unequivocal, and they have shaped how we are looking for the Next Great Thing in Fragile X treatments.
Read moreCompound that Inhibits mGluR5 Corrects Signs of Fragile X in Adult Mice
A study finds that a new compound reverses many of the major symptoms associated with Fragile X syndrome (FXS). The paper is published in the April 12 issue of the journal Neuron, describing the exciting observation that the FXS correction can occur in adult mice, after the symptoms of the condition have already been established. Previous research has suggested that inhibition of mGlu5, a subtype of receptor for the excitatory neurotransmitter glutamate, may ameliorate many of the major symptoms of the disease. This study, a collaboration between a group at Roche in Switzerland, led by Dr. Lothar Lindemann, and Dr. Mark Bear’s MIT lab, used an mGlu5 inhibitor called CTEP to examine whether inhibition of mGlu5 could reverse FXS symptoms.
Read more