Dr. Maquat, 2023 Gruber Genetics Prize winner, discovered NMD, a key surveillance system in the body that protects against mistakes in gene expression. Her groundbreaking research has led to new therapies for Fragile X syndrome.
Read moreResearch Updates
Sex-specific Learning Differences Found in Fragile X Patients, Mouse Model
Girls and women with Fragile X syndrome show different learning impairments relative to boys and men with the disease, a finding that was paralleled in a mouse model of the disease, a study found.
Read moreA Look Back at 2022 and Ahead to 2023 Research Prospects

This holiday season you proved that the adage “actions speak louder than words” is entirely accurate. We sincerely appreciate your gifts in action. FRAXA supporters came out in full force to meet the generous $100,000 challenge offered on Giving Tuesday.
Read more10 Year Vision for Fragile X Research – Dr. Elizabeth Berry-Kravis & Dr. Patricia Cogram

In this video we hear from FRAXA Investigators Dr. Patricia Cogram, Professor at the University of Chile, and Dr. Elizabeth Berry-Kravis, Professor at Rush University Medical Center as they reflect on the progress that has been made and visualize what they see happening in the next 10 years for people living with Fragile X syndrome.
Read moreClinical Trial Results Reported for Phase 3 CONNECT-FX Study of Zygel™

Results have just been published from Zynerba Pharmaceuticals’s phase 3 clinical trial of Zygel™ in the Journal of Neurodevelopmental Disorders. In this trial, 212 children and adolescents aged 3 to 17 years were given Zygel or placebo for 12 weeks.
Read moreFRAXA Volunteer Participates in Peer Reviewed Medical Research Program for the Department of Defense

FRAXA nominated advocate, Jennifer Frobish, recently evaluated research applications submitted to the Peer Reviewed Medical Research Program (PRMRP) of the Congressionally Directed Medical Research Programs (CDMRP).
Read moreWhy Pharma Companies Take on Fragile X, Explained

Research aimed at finding Fragile X syndrome treatments is exploding. Why are so many pharmaceutical and biotech companies investing in this orphan indication? FRAXA chief scientific officer Dr. Michael Tranfaglia explains the many reasons Fragile X is such a hot topic.
Read moreHow FRAXA Prioritizes Research, Explained

Dr. Mike Tranfaglia explains how FRAXA prioritizes research and the importance of looking at research from multiple angles. “It’s not either-or. It’s not we have a definitive treatment or we have a new drug treatment or we have a repurposing treatment. We can have all of those things, mixed or matched, in a personalized medicine kind of way and I think that’s what we’re headed for.”
Read moreAggression, Other Fragile X Behaviors Tend to Ease Over Teenage Years

Behavioral problems such as hyperactivity and aggression are generally more frequent in younger children with Fragile X syndrome, becoming less common as they grow through adolescence and journey toward adulthood, researchers report.
Read moreWhat FRAXA Is Excited about in the Upcoming Fragile X Research Grants, Explained

Dr. Mike Tranfaglia shares what FRAXA is excited about as we work through reviewing all of the submitted Fragile X research grant applications. We find it especially exciting that so many new clinical trials are starting right now, as our major emphasis is getting the drugs and other treatment strategies that we have tested in the Fragile X mouse model to patients in clinical trials.
Read moreGordon Research Conference for Fragile X and Autism-Related Disorders, Explained

The Fragile X and Autism-Related Disorders Gordon Research Conference is a biannual event that provides an international forum for the presentation and discussion of frontier research in these conditions. Dr. Mike Tranfaglia explains why this is the premier conference for researchers and the scientific community.
Read moreRecruiting: Clinical Study of Non-Invasive EEG for Children Ages 2-7

Dr. Carol Wilkinson, MD PhD, and Dr. Charles Nelson, PhD, at Boston Children’s Hospital are recruiting children ages 2-7 years with Fragile X syndrome to participate in a study of brain differences using non-invasive EEG.
Read moreCorrecting Fragile X Syndrome Deficits by Targeting Neonatal PKCε Signaling in the Brain

With this $90,000 grant from 2017-2018, Dr. Banerjee’s team has shown that enhancing PKCε can correct brain development and abnormal behaviors in Fragile X knockout mice and had their findings published in PubMed.
Read morePotential Upcoming Advances in Fragile X Research

Dr. Peter Kind, Director of the Patrick Wild Centre and Professor of Developmental Neuroscience at the University of Edinburgh, and Dr. Nahum Sonenberg, James McGill professor of biochemistry at McGill University, share their optimism about the next 10 years of Fragile X research. They discuss where they think the next big discoveries will emerge.
Read moreNeurodevelopmental Drug Development Summit Presentation

FRAXA president and co-founder, Katie Clapp was one of three patient advocacy leaders invited to kick off the Neurodevelopmental Drug Development Summit with a presentation on Fragile X, and FRAXA Scientific Advisor, Dr. Elizabeth Berry-Kravis also presented lessons learned from clinical trials in Fragile X Syndrome.
Read moreReactivating the Fragile X Gene in Young Mice Reverses Symptoms
A new FRAXA-funded research project offers hope that Fragile X syndrome could be treated by reactivating the gene which is shut down in people with the syndrome. Researchers at the University of California, Riverside report that they were able to reduce FXS symptoms by inserting the FMR1 gene into the brains of very young mice.
Read more10 Year Vision for Collaborations That Transform Fragile X and Autism Research

The future offers hope for people living with Fragile X syndrome. Collaborations between the Fragile X community and other disability organizations help to provide understanding and advancement of research to bring effective treatments to families. FRAXA’s Dr. Mike Tranfaglia talks with Autism Science Foundation’s Allison Singer about the importance of their collaboration as we look forward to the next 10 years.
Read moreNew Fragile X Clinical Trial Announced by Healx

Healx’s AI-driven approach makes finding the right combination therapies more efficient, cost-effective, and rapidly ready for testing at FRAXA-DVI. It was this process that has brought Healx to its recent announcement sharing that it has received Investigational New Drug (IND) approval from the US Food and Drug Administration (FDA) for the Phase 2a clinical study of HLX-0201 (sulindac, an FDA-approved drug).
Read moreCorrecting the Brain’s Emotional Memory Center
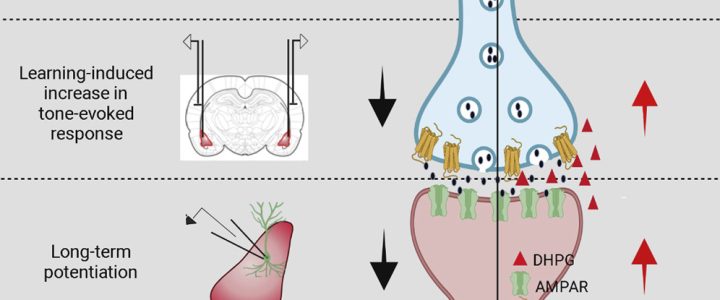
Cell Reports has published the results of the study “Correction of Amygdalar Dysfunction in Rat Model of Fragile X Syndrome” by FRAXA Investigator Dr. Sumatra Chattarji and his team at the National Centre for Biological Sciences in Bangalore, India. The researchers investigated the synaptic basis of deficient conditioned fear and its reversal in Fragile X syndrome rats.
Read moreMaking Drug Development Efficient Through Community-Based Collaboration

FRAXA co-founders Katie Clapp and Mike Tranfaglia, spoke virtually at the 5th Pharma Pricing Reimbursement and Market Access 2021 conference.
In this session, facilitated by Nadia Bodkin, PharmD, MS, from the Rare Advocacy Movement, Katie and Mike were joined by Christopher U. Missling, PhD, President and CEO of Anavex Life Sciences Corp. to discuss the collaboration between FRAXA and Anavex as a case study example to help raise awareness amongst others in the rare disease industry of these types of collaborations between advocacy and industry.
Read more2021 Fragile X Research Grants Funded by FRAXA Research Foundation

Each year, FRAXA funds a diverse portfolio of research. Our FRAXA Fellowships are seed funding for the future, the feedstock for the Fragile X treatment development pathway. While we are looking to promote as many promising new approaches as possible, prominent themes emerge each year, as scientists around the world tackle previously neglected areas.
Read more20 Years of Advancing Fragile X Research: Progress Toward a Cure

Dr. Mark Bear joined the Fragile X field in 1999 when he received a research grant from FRAXA Research Foundation. At the time, we recognized the symptoms of Fragile X, and we knew its cause: a single missing protein. But we knew very little else. Dr. Bear traces the discoveries that now give us great optimism of finding effective treatments and ultimately a cure for Fragile X.
Read moreBrain Organoids, Moving Fragile X Research Forward

There are many ways research produces discoveries, and all of them include a process of steps that build on each other. When an exciting new avenue appeared with potential for Fragile X syndrome, FRAXA stepped up to fund it. We now see the results of this grant and are excited to share them with you. The importance of different types of models have been shared and discussed over many years. We are now adding a “brain organoid” model to this group, and the potential behind it is really exciting.
Read moreTetra’s Fragile X Clinical Trial – The Most Successful So Far

Dr. Mark Gurney, CEO of Tetra Therapeutics, discusses how one of the earliest clues to the biology of Fragile X led to the most successful Fragile X clinical trial to date. FRAXA and Tetra began working together after a key FRAXA-funded study caught the attention of Dr. Gurney. Through the FRAXA Drug Validation Initiative, Dr. Patricia Cogram was able to conduct preclinical validation experiments with Tetra’s lead compound in record time, paving the way for clinical trials.
Read morePromising Results of Preclinical Study of ANAVEX®2-73

We are excited to share that Anavex Life Sciences announced today that preclinical data of the ANAVEX®2-73 (blarcamesine) study in Fragile X syndrome were published in the peer-reviewed journal, Scientific Reports.
Read more
