We are excited to share that Anavex Life Sciences announced today that preclinical data of the ANAVEX®2-73 (blarcamesine) study in Fragile X syndrome were published in the peer-reviewed journal, Scientific Reports.
Read moreResearch Updates
Synaptogenix Announced Intention to Launch a Fragile X Clinical Trial with Bryostatin

One of the most exciting kinds of work that FRAXA does is following the journey of an experimental new treatment until it is ready for trials in people with Fragile X. From an initial idea, through the development process, to clinical trials, FRAXA helps out all along the way. From an initial idea, through the development process, to clinical trials, FRAXA helps out all along the way. The recent announcement by Synaptogenix is a great example of how FRAXA funding and use of FRAXA-DVI can accelerate research on Fragile X.
Read moreFragile X Syndrome: In Pursuit of a Cure Webinar

A global webinar titled “Fragile X Syndrome: In Pursuit of a Cure,” took place on July 22, 2021 to commemorate World Fragile X Day. This complimentary event is co-organized with WuXi AppTec. We are delighted that more than 5,000 registered from more than 50 countries worldwide, coming together to raise awareness of Fragile X, and to foster collaborations towards effective treatments and ultimately a cure.
Read moreDrug Tolerance in MGluR5 Clinical Trials – Dr Patrick McCamphill 1:1 with FRAXA

We have long suspected that the clinical trials of mGluR5 blockers from Novartis and Roche failed because the drug triggered tolerance, losing effect over time. With a $90,000 grant from FRAXA, Dr. Patrick McCamphill, a Postdoctoral Fellow in the MIT lab of Dr. Mark Bear, is investigating. He does indeed find tolerance, and now he is looking for ways to overcome it.
Read morePivotal Phase 3 Trial of Zygel in Severe Fragile X Possible This Year

Zynerba Pharmaceuticals reported receiving advice from the U.S. Food and Drug Administration (FDA) on the design of an upcoming Phase 3 clinical trial meant to confirm previous trial findings supporting Zygel as a cannabidiol treatment in a specific subset of Fragile X syndrome patients. The new trial, called RECONNECT, is expected to launch before October, and will mainly enroll children and adolescents with a complete (100%) methylation of FMR1, the gene mutated in Fragile X.
Read moreTetra Releases Full Results of FRAXA-Funded Clinical Trial of PDE4D Inhibitor

Today, Tetra Therapeutics published the full results of its PDE4D trial published the full results to their announcement. Now having reviewed the full results, FRAXA can confidently say that the PDE4D drug trial gives hope to patients and families that Fragile X Syndrome is a treatable disorder, and this particular drug can improve intellectual disability.
Read moreParkinson’s Therapy May Hold Promise for Fragile X

A study funded by FRAXA in Italy has encouraging results for people with Fragile X: drugs that block adenosine receptors (A2A) reversed signs of Fragile X in a mouse model.
“One of the most intriguing things about this study is that it points to an entire drug class (not just the one drug used) as potentially therapeutic for Fragile X. Many available compounds block A2A receptors, and we know they are safe and effective.
Read moreThe Why and How of FRAXA’s Work to Find a Cure for Fragile X

Thank you Talk Fragile X for having FRAXA’s cofounder Katie Clapp as a guest on your podcast! It was a pleasure to share why FRAXA got started and our motivation for finding effective treatments and ultimately a cure for Fragile X syndrome.
Read moreBeneath the Surface of Fragile X Syndrome: Study Sheds Light on What’s Happening in Nerve Cells
This FRAXA-funded project has turned up some surprising results. At first, it might seem Kurosaki and Maquat have found yet another cellular process which is malfunctioning in Fragile X. But this finding is intimately related to previous findings of abnormal protein synthesis and misregulated transcription in Fragile X. FMRP (the protein lacking in Fragile X syndrome) is involved in chaperoning messenger RNAs within cells to active sites, and in controlling their translation into many different proteins. Some of these proteins are transcription factors, which feed back to the nucleus to control gene expression.
Read moreFragile X Research Funding of $1 Million Offered for 2021

FRAXA plans to fund $1 million for a generous number of Fragile X research grants and fellowships in 2021. Our mission is to find specific treatments and ultimately a cure for Fragile X syndrome. We aim to bring practical treatment into current medical practice as quickly as possible; we prioritize projects that have a clear practical application and the results of which will be shared in a timely fashion.
Read moreIntegrating Human and Mouse Studies in Fragile X Syndrome – an NIH Center Approach

Presentations by:
Craig Erickson – Translational medicine and mechanistic studies of brain neurophysiology in Fragile X Syndrome: A NIH Center Overview
Ernest Pedapati – Network Mechanisms, Biomarkers, and Pharmacology of Fragile X Syndrome in Humans
Devin Binder – Network Mechanisms of Neurophysiology and Behavior in mouse models of Fragile X Syndrome
Kimberly Huber – FMRP Regulation of local and long-range neocortical circuits in the mouse: Links with EEG phenotypes
Developing Arbaclofen for Fragile X – Dr. Mark Bear 1:1 with FRAXA

Seven years ago, arbaclofen (STX209) was pulled from development, disappointing families around the US. Now MIT professor and FRAXA Investigator Dr. Mark Bear has founded Allos Pharma to bring it back. Dr. Bear sat down with FRAXA co-founder Katie Clapp to share the story and next steps.
Read moreBryostatin-1 in Long-term Use Seen to Arrest Fragile X Symptoms in Mouse Model
Long-term, but not short-term, treatment with bryostatin-1 — Neurotrope’s lead investigational therapy — arrested such behavioral and cognitive symptoms as hyperactivity, difficulties with daily life activities, and learning and memory deficits in a mouse model of Fragile X syndrome.
Read moreAllos Pharma Revives Arbaclofen After 7 Years: New Hope for Fragile X Syndrome

Experience the revival of arbaclofen as Allos Pharma Inc launches a new development program, providing renewed hope for the Fragile X community. Discover the impact of this experimental drug and the determination of those who never gave up.
Read morePositive Results Reported in Phase II Fragile X Clinical Trial of PDE4D Inhibitor Zatolmilast from Tetra Therapeutics

Today, Tetra Therapeutics announces the first unequivocally positive phase 2 clinical trial in Fragile X syndrome, press release below. The results do not depend on carving out a subset of patients or post hoc analysis.
Read moreOvercoming the Placebo Effect in Fragile X Clinical Trials

In a placebo-controlled clinical trial, some participants are given an experimental medication, while others are given a placebo. Participants do not know whether they are taking medicine or placebo. In theory, this can allow researchers to rule out the placebo effect by comparing outcomes among the two groups. But, per Wexler (2020) “having a strong placebo effect can obscure any real effect of the therapy being investigated”.
Read moreZygel May Improve Behavior in Children With Severe Fragile X, Trial Data Suggest

Zynerba presented clinical trial results for Zygel at a recent neurology conference. Zygel, an experimental cannabidiol (CBD) gel, may reduce behavioral abnormalities in children with Fragile X syndrome who have more severe disease.
Read moreHealx Drug Repurposing Programme for Fragile X Syndrome

David Brown, MD, PhD, Ivan Angulo-Herrera, PhD and Anthony Hall of Healx present about the Drug Repurposing Programme for Fragile X syndrome.
Read moreYale Researcher Dr. Elizabeth Jonas 1:1 with FRAXA

We talk with Dr. Elizabeth Jonas, Professor of Internal Medicine and Neurology at Yale School of Medicine, about her new research suggesting that leaky mitochondria cause some symptoms of Fragile X syndrome. Leaky membranes may also be involved in Parkinson’s Disease and other diseases.
Read moreMechanisms and Biomarkers of Sensory Hypersensitivity in the fmr1 Knockout Mouse

In this Fragile X research webinar we hear from Devin K. Binder, MD, PhD, Professor, University of California at Riverside Medical School and Khaleel Razak, PhD, Professor, University of California at Riverside as they present about Mechanisms and Biomarkers of Sensory Hypersensitivity in the fmr1 Knockout Mouse.
Read moreAripiprazole (Abilify) in the Treatment of People with Fragile X: An Anecdotal Account

The aim of this article is to discuss the use of Abilify (generic name: aripiprazole) as a treatment for people with Fragile X syndrome (FXS). As an “off-label” prescription, Abilify targets behaviors such as irritability, aggression, self-injury and severe tantrums.
Read moreBrain Organoids and Therapeutic Development for Fragile X and Other Rare Diseases

This is the first in a series of webinars focused on current topics in Fragile X research. In this webinar we hear from Alysson R. Muotri, PhD, Professor at University of California San Diego Stem Cell Programand Fabio C. Tucci, PhD, Chief Operating Officer and co-founder at Epigen Biosciences, Inc.
Read moreTrofinetide Clinical Trial Results Published
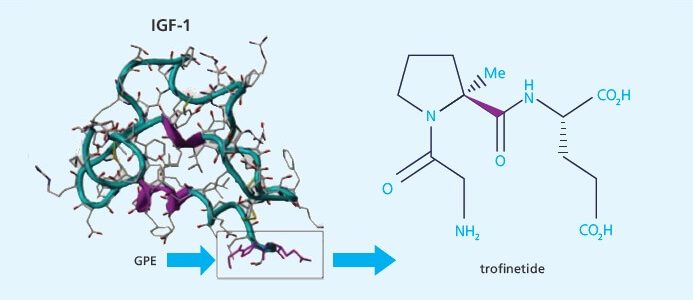
New Zealand-Based Biotech Neuren Pharmaceuticals Has Published Successful Phase 2 Fragile X Clinical Trial. Trofinetide, given to adolescent and adult males with Fragile X syndrome, was shown to be generally safe and was well-tolerated. It also showed preliminary evidence of efficacy. This trial validated a new design which can be used in future trials.
Read moreScientists Find a New Way to Reverse Symptoms of Fragile X
FRAXA Investigator and MIT Professor Mark Bear and his colleagues have identified a valuable new target for Fragile X therapeutics: GSK3 alpha. Several FRAXA research teams previously identified GSK3 beta as a treatment target for Fragile X. The catch is that, so far, GSK3 beta inhibitors have proven too toxic for regular use. Dr. Bear’s new discovery opens up the possibility of developing more selective compounds with less toxicity and fewer side effects. Interestingly, lithium inhibits both GSK3 versions – alpha and beta.
Read moreCompanies Move to Advance Potential Cognitive Treatment for Fragile X

Tetra Therapeutics and Shionogi announced plans to expand their partnership supporting BPN14770, a treatment candidate for disorders marked by cognitive and memory deficits, including Fragile X syndrome and Alzheimer’s disease. The agreement builds on an earlier collaboration between the two companies, and aims to further accelerate BPN14770’s development and potential marketing. It is currently in clinical testing in both Fragile X and Alzheimer’s patients.
Read more
