Discover how a $100,000 FRAXA grant awarded to Cincinnati Children’s Hospital is advancing Fragile X research by simplifying EEG technology for home use, improving clinical trial accessibility and efficiency.
Read moreClinical
Somatosensory Processing as a Therapeutic Target for Fragile X Syndrome
Awarded a FRAXA Research grant, Dr. Andrew Stanfield, Dr. Leena E. Williams, and Dr. Damien Wright are set to explore somatosensory processing (sense of touch) in Fragile X syndrome at the University of Edinburgh. Their aim? A noninvasive touch test that could set the stage for future clinical trials in FXS.
Read moreDevelopmental Motor Phenotype in Fragile X Syndrome

One of the lesser known signs of Fragile X is unsteady walking. This is also very easy to evaluate in the clinic: no blood tests are required! With a $100,000 grant from FRAXA Research Foundation, this team will develop objective new outcome measures of gait for future treatment trials and also to see if exercise could improve other symptoms of Fragile X.
Read moreFragile X Clinical Trial of New PDE4D Inhibitor from Tetra

With a $200,043 grant from FRAXA Research Foundation, Dr. Elizabeth Berry-Kravis completed a successful Phase 2 clinical trial of a PDE4 inhibitor for adult men with Fragile X syndrome. This trial treated 30 males, 18-45 years of age with a new PDE4D allosteric inhibitor from Tetra Discovery Partners using a crossover design, so that everyone got active drug for part of the time and placebo for part of the time.
Read moreRecruiting: Clinical Study of Non-Invasive EEG for Children Ages 2-7

Dr. Carol Wilkinson, MD PhD, and Dr. Charles Nelson, PhD, at Boston Children’s Hospital are recruiting children ages 2-7 years with Fragile X syndrome to participate in a study of brain differences using non-invasive EEG.
Read moreLovamix: Clinical Trial of Combined Treatment of Minocycline and Lovastatin in Fragile X Syndrome

With a $66,714 grant from the FRAXA Research Foundation awarded over 2015-2017, Dr. Francois Corbin at the Universite of Sherbrooke will test the safety and synergistic effects of lovastatin and minocycline in patients with Fragile X syndrome.
Read morePurposeful and FRAXA Partnership Leads to Clinical Trial

Can a combination of drugs make a meaningful difference for people with Fragile X? A new clinical trial is going to find out. 15-20 adult men with Fragile X will be included in this trial to test the effects of an available drug and a nutritional supplement taken together.
Read moreLink Between Lipid Profile, eCBome System and Gut Microbiome in Fragile X Syndrome
Why does obesity challenge so many people with Fragile X? Dr. Caku’s team thinks changes in the gut are the culprit. This team has found that Fragile X syndrome causes changes in the tiny organisms that live in our gut. They believe that these abnormalities cause changes in the brain which impair learning and behavior.
Read morefNIRS to Measure Treatment Response in Young Children with Fragile X

FRAXA Research Foundation has awarded a $90,000 research grant to Dr. Craig Erickson and Dr. Elizabeth Smith at Cincinnati Children’s Hospital to test functional near-infrared spectroscopy (fNIRS), in children who have Fragile X syndrome. fNIRS is safe, non-invasive, and easily-tolerated. It uses light sources and sensors on the scalp to build a heat map of the brain in action.
Read moreClinical Trial of Metformin for Fragile X Syndrome

Metformin is commonly prescribed to control high blood sugar in type 2 diabetes. With a $50,000 grant from FRAXA Research Foundation, Dr. Artuela Çaku and Dr. Francois LePage are conducting an open-label clinical trial of metformin for children and adults with Fragile X syndrome, at the University of Sherbrooke in Canada.
Read moreFragile X Clinical Trial of AZD7325 in Adults

With a $51,000 grant from FRAXA Research Foundation, Dr. Craig Erickson conducting a double-blind, placebo-controlled clinical trial of AZD7325 in adults ages 18-50 with Fragile X syndrome at Cincinnati Children’s Hospital. The compound being studied is an investigational new drug from AstraZeneca that targets GABA (A) receptors.
Read moreBrain Imbalance Target of Dr. Erickson’s New Clinical Trial

According to Dr. Erickson, AZD7325 is a drug that selectively boosts GABA neurotransmission in the brain. GABA is the primary neurochemical in the brain that blocks brain activation. GABA activity is in balance in the brain with Glutamate activity, which is the primary neurochemical that causes brain activation. In Fragile X, GABA activity is insufficient and glutamate activity is excessive, likely causing brain activity to be out of balance. AZD7325 attempts to correct parts of this imbalance by boosting the insufficient GABA activity in the brains of people with Fragile X.
Read moreClinical Trial of Ganaxolone in Patients with Fragile X Syndrome

With a $90,000 grant from FRAXA Research Foundation funded during 2014-2015, Dr. Frank Kooy and colleagues at the University of Antwerp are conducting a double blind crossover trial of ganaxolone in patients with Fragile X syndrome. Results of this study were mixed (see Marinus: Results from Phase 2 Exploratory Clinical Study Support Continued Development of Ganaxolone in Fragile X Syndrome.)
Read moreNeural Markers of Fragile X: A Powerful New Tool for Clinical Trials
Once the neural marker is identified for a particular challenge, such as kids with poor language versus good language, neural markers can be measured during drug and behavioral therapy trials to see if a child is improving based on objective biological measures.
Read moreTrial and No Error: Better Outcomes for Clinical Trials in Fragile X Syndrome

Johns Hopkins researcher Christina Timmerman, PhD, searches for a less subjective method to determine if a drug is working in patients with Fragile X syndrome. Many parents of children with Fragile X syndrome were crushed when promising drug trials were unexpectedly stopped a few years ago because subjective behavior-based outcome measures did not justify continuing the trials. The strong feelings linger today. If all goes well with Christina Timmerman’s research, future drug trials may be able to continue with additional metrics for assessment, until there are advanced treatments or even a cure for Fragile X syndrome.
Read moreDouble Down: Fragile X Clinical Trial Combines Two Available Drugs
If all the science world’s a stage, Fragile X researchers are more than merely players. They are center stage. So believes Francois Corbin, MD, PhD, professor, Université de Sherbrooke, Canada, who directs the university’s Fragile X Clinic. Corbin, who has received more than $100,000 in FRAXA support since 2012, is leading a pilot randomized Phase II trial, exploring the tolerability and the synergistic effect of a combined therapy.
Read moreAnalysis of Developmental Brain Dysfunction in Families
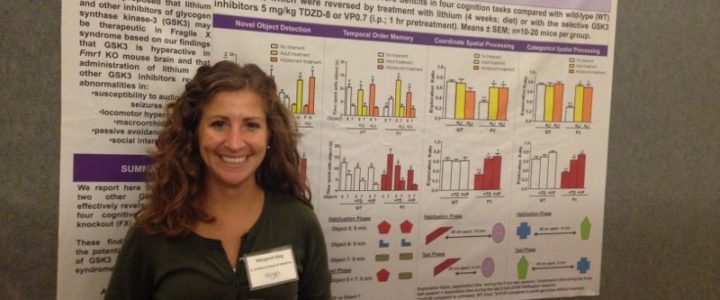
FRAXA Research Foundation is proud to make a grant of $90,000 over 2014-2015 to Margaret King, PhD. The goal of this project is to identify new approaches to clinical trial design for Fragile X pharmaceuticals.
Read morePhase 1 Clinical Trial of Mega Green Tea Extract in Fragile X Syndrome

With a $124,000 grant from the FRAXA Research Foundation from 2012-2014, Dr. Mara Dierssen and Dr. Rafael de la Torre conducted preclinical studies in Fragile X knockout mice and a clinical trial in Fragile X patients using Mega Green Tea Extract, which contains 45% by weight epigallocatechin gallate (EGCG).
Read moreSocial Behavior as an Outcome Measure for Fragile X Clinical Trials

One of the features of the Fragile X mouse model which is relevant to the human Fragile X syndrome (and autism) is social behavior. Several tests show consistent social behavioral abnormalities in the Fragile X mouse model. With a $140,000 grant from FRAXA Research Foundation in 2012-2013, Dr. Willemsen at Erasmus University used social behavior tests to measure the effectiveness of several drug strategies.
Read moreDevelopment of a Novel GABA-A Agonist in Fragile X Syndrome

Of the many genes known to be regulated by FMRP, the gamma-aminobutyric acid receptor A (GABA(A)), is gaining attention as a potential target for the treatment of FXS. Mounting evidence suggests decreased expression and functioning of GABA(A) is involved in the pathophysiology of FXS. Non-selective GABA(A) agonism in animal models of FXS has been associated with normalization of morphological features, GABA(A) expression, and behavior. However, the clinical use of these agents in Fragile X is associated with unwanted side-effects, such as sedation, dulling of cognition, and occasional paradoxical agitation, which limits their use.
Read morePilot Clinical Trial of Lithium in Fragile X Shows Promising Results

With a $65,000 grant from FRAXA Research Foundation in 2005, Dr. Berry-Kravis at the Rush University Medical Center conducted a pilot clinical trial of lithium in 15 patients with Fragile X syndrome. Results published.
Read moreEncouraging Results from First Trial of Minocycline in Fragile X

With a $40,000 grant from FRAXA, Dr. Carlo Paribello and his team at the Surrey Place Centre Fragile X clinic in Toronto, Ontario, ran an open label trial to see if minocycline can improve learning and reduce anxiety and behavioral problems in people with Fragile X. Twenty participants between the ages of 13 and 35 years took minocycline for two months.
Read moreNeuromotor Outcome Measures for Clinical Trials in Fragile X Syndrome

With a $35,000 grant from FRAXA Research Foundation, Dr. Nicole Tartaglia from the University of Colorado Denver and Tracey Stackhouse aimed to develop neuromotor outcome measures for use in clinical trials in FXS, and to contribute to a deeper understanding of the neuromotor issues involved in FXS. This collaborative project was completed at the two sites of the Colorado Fragile X Clinic: The Children’s Hospital and Developmental FX. Dr. Nicole Tartaglia is the Medical Director of the Fragile X Clinic at The Children’s Hospital of Denver. Tracy Murnan Stackhouse, MA, OTR is the co-founder of the Developmental & Fragile X Resource Centre (Developmental FX), a clinic specializing in Fragile X.
Read moreAberrant Behavior Checklist in Fragile X Syndrome

With a $10,000 grant from FRAXA Research Foundation, Dr. Hessl at the University of California at Davis led a collaborative study to analyze the Aberrant Behavior Checklist (ABC) as an outcome measure for children and adults with Fragile X syndrome. Results published.
Read moreClinical Trial of Aripiprazol in Fragile X Syndrome

With a FRAXA Research Foundation grant of $30,000 in 2006, Dr. Erickson conducted a pilot clinical trial of an available medicine, aripiprazole (brand-name Abilify). This was an open-label 12-week trial in 12 people ages 6–25 years with Fragile X. Results were promising, and published: 10 of the 12 participants showed behavioral improvements.
Read more
