Correcting Fragile X Syndrome Deficits by Targeting Neonatal PKCε Signaling in the Brain
Enhancing PKCε in early development normalized oxytocin, AMPAR signaling, and adult behavior in Fragile X mice, highlighting PKCε as a promising therapeutic target.
Climbing 3 Mountains for Fragile X Research
Michael Leonard climbed three mountains in 24 hours to honor his son, Cole, who lives with Fragile X syndrome. A father’s love knows no limits.
Potential Upcoming Advances in Fragile X Research
Dr. Peter Kind, Director of the Patrick Wild Centre and Professor of Developmental Neuroscience at the University of Edinburgh, and Dr. Nahum Sonenberg, James McGill professor of biochemistry at McGill University, share their optimism about the next 10 years of Fragile X research. They discuss where they think the next big discoveries will emerge.
Lovamix: Clinical Trial of Combined Treatment of Minocycline and Lovastatin in Fragile X Syndrome
FRAXA funded the LovaMiX trial of lovastatin + minocycline for Fragile X. 2022 results show safety and support continued study of this dual-target treatment approach.
FRAXA’s Most Successful End-Of-Year Campaign Ever!
Together, we made history! FRAXA’s 2021 campaign was our most successful ever, fueling hope for treatments and a cure for Fragile X.
Neurodevelopmental Drug Development Summit Presentation
FRAXA president and co-founder, Katie Clapp was one of three patient advocacy leaders invited to kick off the Neurodevelopmental Drug Development Summit with a presentation on Fragile X, and FRAXA Scientific Advisor, Dr. Elizabeth Berry-Kravis also presented lessons learned from clinical trials in Fragile X Syndrome.
Reactivating the Fragile X Gene in Young Mice Reverses Symptoms
A new FRAXA-funded research project offers hope that Fragile X syndrome could be treated by reactivating the gene which is shut down in people with the syndrome. Researchers at the University of California, Riverside report that they were able to reduce FXS symptoms by inserting the FMR1 gene into the brains of very young mice.
Meet Jonathan!
Meet #FriendofFRAXA Jonathan! If you would like to nominate someone as a #FriendofFRAXA, we welcome all who have been touched by Fragile X, including friends, grandparents, siblings, professionals and companions alike to become a #FriendofFRAXA with the goal of putting a face to Fragile X for those who may not know someone directly.
10 Year Vision for Collaborations That Transform Fragile X and Autism Research
The future offers hope for people living with Fragile X syndrome. Collaborations between the Fragile X community and other disability organizations help to provide understanding and advancement of research to bring effective treatments to families. FRAXA’s Dr. Mike Tranfaglia talks with Autism Science Foundation’s Allison Singer about the importance of their collaboration as we look forward to the next 10 years.
Patrick’s PALS Still Shines Strong Even in the Face of COVID
Patrick’s PALS 25th Annual Fundraiser raised $140K for FRAXA in 2021, proof that the spirit and generosity behind this event never fade.
16th Annual Fragile X Poker Run Raises nearly $29,000
The 16th Annual Fragile X Poker Run brought friends from 10 states and Israel together, raising $29K for FRAXA research!
GABA-A Receptor in Fragile X Syndrome
Published results from Dr. Frank Kooy reveal how GABAA receptor changes may hold the key to treating Fragile X syndrome.
Characterization of a Novel CYFIP1 – Derived Peptidomimetic Restoring the Dysregulated mRNAs Translation: Toward An Innovative Therapeutic Strategy for FXS
This team is designing tiny “peptidomimetic” drugs that mimic FMRP’s function to rebalance protein production in the brain, aiming to treat Fragile X at its source.
Bruins 50/50 Benefits Fragile X Research
FRAXA volunteers sold 50/50 raffle tickets at the Bruins game, raising $28,040 with the Bruins Foundation, half to FRAXA, half to one lucky fan!
FX-Learn Clinical Trial for Children with Fragile X
Thirteen centers across the US enrolled children with Fragile X in a large-scale clinical trial of Novartis AFQ056. Dr. Elizabeth Berry-Kravis and colleagues aim to show that this targeted treatment — an mGluR5 blocker for Fragile X which failed in previous adult human trials — can be better evaluated by studying effects on learning in young children.
Over $20,000 Raised For Fragile X Research at Callum Cup V
After a year off for COVID, the Callum Cup returned! Millburn FC’s marquee charity match honors Callum Murphy, son of goalkeeper Andrew.
Cannabinoids as a Treatment for Fragile X Syndrome
This team uses EEG to study sensory hypersensitivity in Fragile X. By testing drugs in mice, they aim to find treatments that calm brain overactivity.
Fundraising Never Tasted So Good
Ice cream for a cause! Lancaster Sweet Shoppe donated proceeds to FRAXA, raising $15,205 in honor of JT. Sweet treats, sweeter impact.
New Fragile X Clinical Trial Announced by Healx
Healx’s AI-driven approach makes finding the right combination therapies more efficient, cost-effective, and rapidly ready for testing at FRAXA-DVI. It was this process that has brought Healx to its recent announcement sharing that it has received Investigational New Drug (IND) approval from the US Food and Drug Administration (FDA) for the Phase 2a clinical study of HLX-0201 (sulindac, an FDA-approved drug).
Forbes, “Clinical Trials Expand For AI-Designed Drug To Treat Fragile X Syndrome”
British startup Healx has secured FDA approval for a phase 2a clinical trial of an AI-discovered compound that could help manage the symptoms of the genetic disorder Fragile X syndrome. The start of the trial marks another milestone in the use of artificial intelligence to help find new applications for existing drugs by mining patient records and research databases.
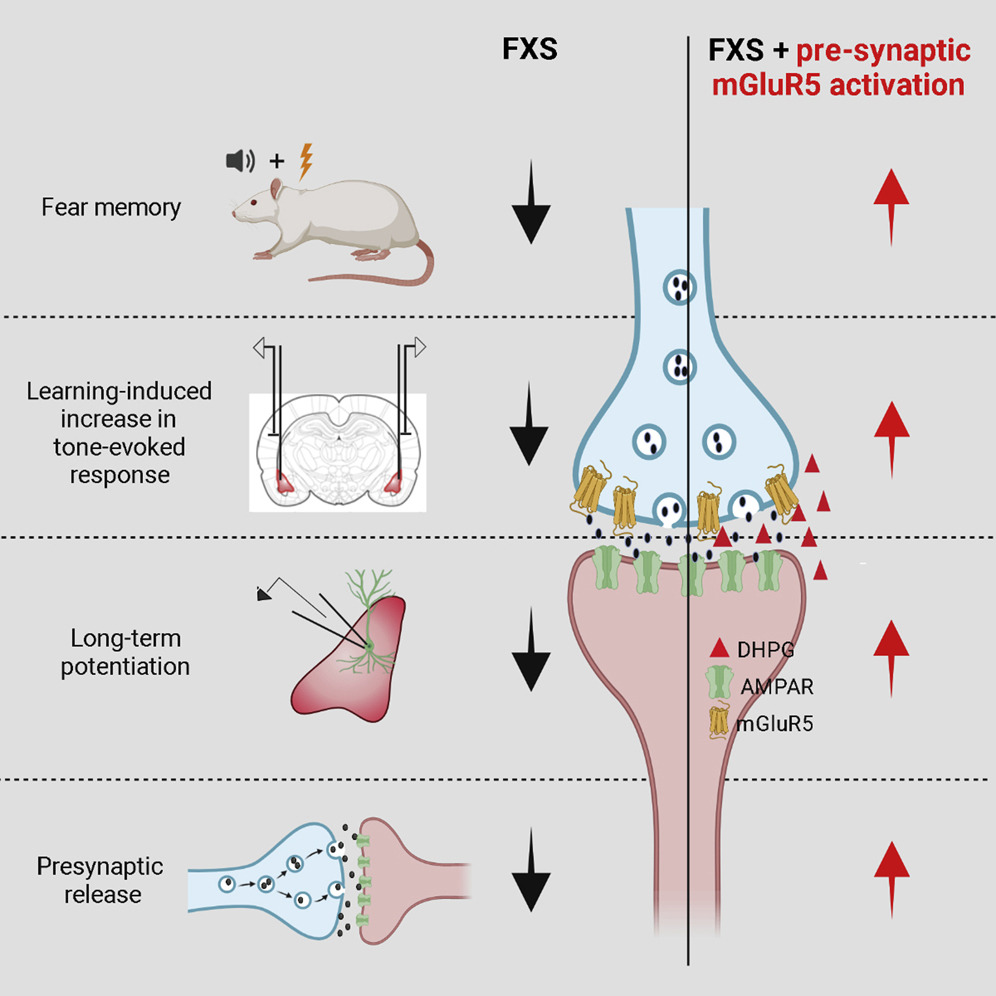
Correcting the Brain’s Emotional Memory Center
FRAXA Investigator Dr. Sumatra Chattarji investigated the synaptic basis of deficient conditioned fear and its reversal in Fragile X syndrome rats. Results published.
Purposeful and FRAXA Partnership Leads to Clinical Trial
AI and FRAXA-DVI identified a drug + supplement combo that reversed all Fragile X symptoms in mice. A clinical trial tested this in adults with Fragile X.
Making Drug Development Efficient Through Community-Based Collaboration
FRAXA’s partnership with Anavex shows how early collaboration between patient advocates and pharma can accelerate drug development for Fragile X and rare diseases.
Meet Matthew!
Meet #FriendofFRAXA Matthew! He is in his final year of school and has always been enthusiastic about his education. He has faced each day with a positive attitude while being friendly and outgoing. Those who meet Matt say he leaves a lasting impression on them. He has been called “The Mayor”, as everyone knows and remembers Matthew where ever goes.