Correcting Fragile X Syndrome Deficits by Targeting Neonatal PKCε Signaling in the Brain
Ernest Pedapati, MD
Co-Principal Investigator
Christina Gross, PhD
Co-Principal Investigator
Craig Erickson, MD, PhD
Collaborator
Lindsay Beasley
FRAXA Fellow
Cincinnati Children’s Hospital
Cincinnati, OH
2019-2021 Grant Funding: $90,000
Summary
With this $90,000 award from FRAXA, Drs. Ernest Pedapati, Christina Gross, and student Lindsay Beasley pursued preclinical gene therapy approaches using AAV (adeno-associated virus) vectors for treating Fragile X syndrome at Cincinnati Children’s Hospital.
The Science
By Ernest Pedapati, MD, MS and Christina Gross, PhD
With the support of FRAXA, master’s student Lindsay Beasley, under the mentorship of Ernest Pedapati, MD, MS and Christina Gross, PhD, will investigate preclinical gene therapy approaches to restoring FMRP activity. Her work is part of a larger novel clinical therapeutics program in Fragile X syndrome at Cincinnati Children’s Hospital, led by Craig Erickson, MD, seeking to develop next generation biomarkers and treatments to address the core features of the disorder.
Recent headlines in diseases such as Spinal Muscular Atrophy have demonstrated the feasibility and potential of viral mediated protein replacement in single gene disorders. The absence of FMRP in the Fragile X brain leads to many unwanted downstream effects which presents significant challenges for drug development. Thus, protein replacement of FMRP in the brain may offer a unique strategy to comprehensively restore function across multiple neural pathways.
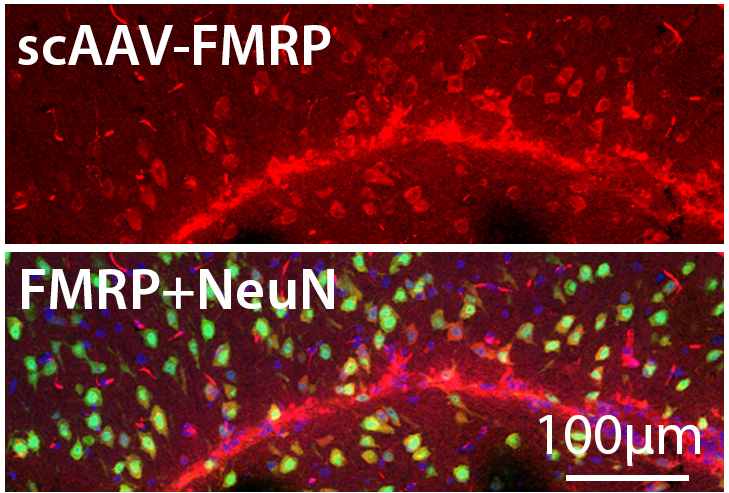
Ms. Beasley will express a series of AAV (adeno-associated virus) vectors, each expressing FMRP, in the mouse brain to identify key design characteristics of those vectors that affect safety, efficacy, and spread of protein expression. Dr. Durgesh Tiwari in Dr. Gross’ lab will train Ms. Beasley to perform conventional primary endpoints in mice studies, including molecular, cellular, and behavioral tests. However, we will also examine the effects of protein expression on mouse EEG signals which have been found to be altered in similar fashion to humans affected by Fragile X syndrome through our collaboration in a larger National Institutes of Health U54 Fragile X Center.
Ms. Beasley will receive additional training by Carrie Jonak through Devin Binder’s lab at the University of California Riverside, to extend our current single lead mouse EEG techniques into implanting 30-channel multi-electrode grids to provide sophisticated signals similar to human dense array EEG. The discovery of a neural “signature” indicating brain activity of a successful host or treatment response is a key goal of this work.
These experiments, along with others at our institution, are laying critical groundwork for the potential adoption of these powerful technologies to one day bring into the clinic.