This changes everything!
In a paper published yesterday, "Antisense oligonucleotide rescue of CGG expansion–dependent FMR1 mis-splicing in fragile X syndrome restores FMRP" in the Proceedings of the National Academy of Sciences of the USA, FRAXA Research Foundation principal investigator Dr. Joel Richter and FRAXA Fellow Dr. Sneha Shah at UMass Chan Medical School and FRAXA investigator Dr. Elizabeth Berry-Kravis at Rush University Medical Center, describe a discovery that completely changes our understanding of Fragile X syndrome.
Redefining Fragile X Syndrome
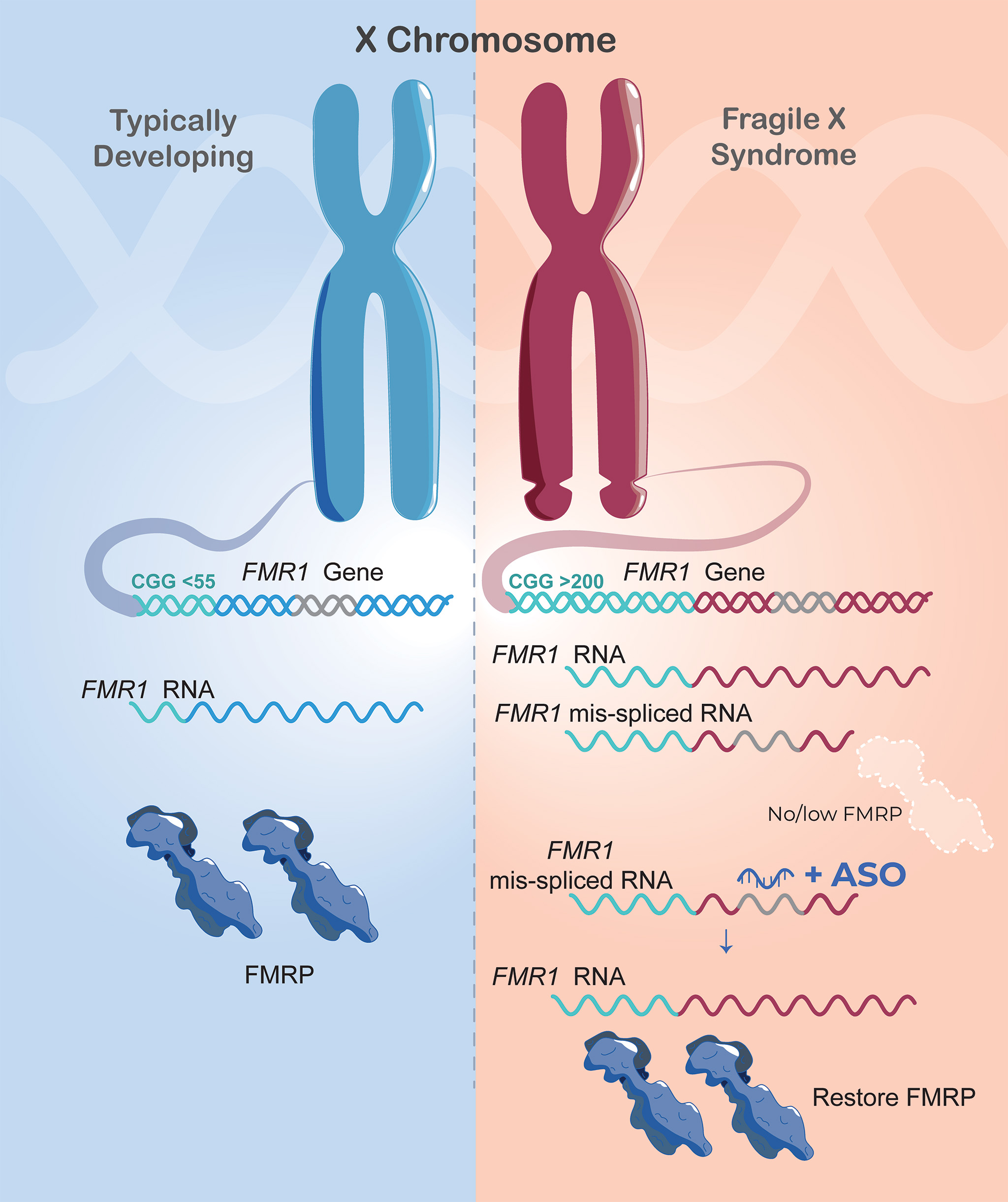
Conventional wisdom holds that in people with full CGG expansion mutations of the Fragile X gene, FMR1, the gene is completely shut down. The Richter lab discovered that this is not always true. In most cases, the gene produces a messenger RNA that is abnormally spliced and - because of this abnormal splicing - no protein is made.
They went on to show that if this abnormal messenger RNA splicing event is blocked using an ASO (explained below), then the FMR1 gene produces normal messenger RNA which in turn produces normal protein!
A Serendipitous Discovery
This project began with rather modest goals, with a FRAXA grant to Drs. Richter and Shah and their collaborator Dr. Elizabeth Berry-Kravis to study alternative splicing of messenger RNAs in the blood of patients with Fragile X syndrome. The Richter lab had observed that the messenger RNAs from many different genes were mis-spliced in Fragile X, and they thought that this might be useful as a blood-based biomarker.
They set about the task of attempting to correlate these abnormal splicing events with the clinical features of individual patients. In the process of documenting the many abnormal splicing events, they discovered something unexpected and extremely interesting: one of the RNAs that was mis-spliced in most of the blood samples from Fragile X patients was encoded by the Fragile X gene, FMR1. Astonishingly, the gene was active!
As the team studied this phenomenon further, they saw something even more remarkable. Most of these individuals who were producing significant amounts of the normal FMR1 Messenger RNA, still were not producing much, if any, protein. This was surprising, to say the least, and led the team to hypothesize that if this abnormal splicing event could be corrected, they might be able to restore production of the protein (FMRP). If so, then this could form the basis for a therapeutic strategy that heretofore is unknown in Fragile X syndrome.
ASOs to the Rescue: A New Approach to Fragile X Treatment
One way to alter RNA splicing is to create an antisense oligonucleotide (ASO), a short piece of DNA with a complementary sequence which will bind to the target messenger RNA. A number of other brain diseases are currently being treated clinically with ASO-based therapeutics, and this is an area that has attracted a huge amount of biotechnology investment. In further collaboration with UMass Chan Medical School ASO expert Dr. Jonathan Watts, they created ASOs which could successfully inhibit improper FMR1 splicing and rescue proper FMR1 splicing. The result was production of significant amounts of FMRP in these patient-derived cells.
This serendipitous discovery represents an exciting possibility for a new therapy to reactivate the Fragile X gene! Last month, FRAXA convened experts in the field at the Special Banbury Meeting on Curative Therapies to advance this discovery and other methods for definitive therapeutics.
This discovery was only possible because of an exceptionally generous FRAXA grant that paid for a large amount of RNA sequencing. It is not possible to recognize all of these abnormal splice variants without very detailed, expensive work. This is something that investigators must be looking for in order to find, and this probably explains why it has not been recognized previously.
The gene is apparently active in most but not all cases. Just 70 to 75% of blood samples tested so far contained this abnormally spliced messenger RNA, and the ASO would be able to restore FMRP production in the cases where this was found. However, the scientists took cells that reflect the minority of patients who were not producing any FMR1 messenger RNA and treated them with a DNA demethylating agent, along with the ASO, which then did result in production of FMRP. This implies that the general approach could be useful in nearly all cases.
This discovery explains why many previous attempts to reactivate the Fragile X gene via demethylation were unsuccessful. This abnormally spliced messenger RNA is the predominant form of the FMR1 messenger RNA in Fragile X patients, and until it is removed from the cell, the normal message cannot be produced in sufficient amounts and translated into protein.
It is quite likely that this discovery will be commercialized in the very near future and serve as the basis for a new company to develop definitive therapeutics for Fragile X syndrome.
Written by
Michael Tranfaglia, MD
Medical Director, Treasurer, Co-Founder
Dr. Michael Tranfaglia is Medical Director and Chief Scientific Officer of FRAXA Research Foundation, coordinating the Foundation’s research strategy and working with university and industry scientists to develop new therapeutic agents for Fragile X. He has a BA in Biology from Harvard University and an MD from the University of North Carolina at Chapel Hill. His son Andy has Fragile X syndrome.