Fragile X syndrome research shows PDE inhibitors may improve brain function and behavior, with promise for related neurodevelopmental disorders.
Read moreAlzheimer’s
Shionogi’s EXPERIENCE Phase 3 Clinical Trial of Zatolmilast in Fragile X Syndrome
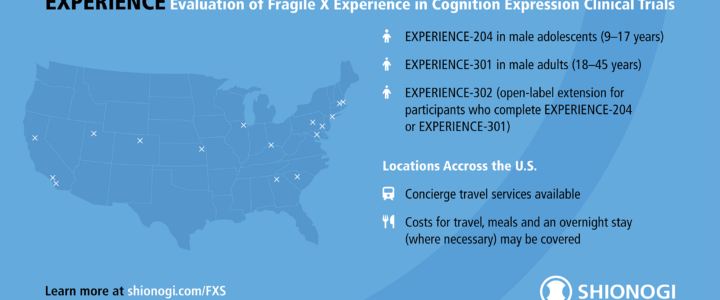
Shionogi’s EXPERIENCE clinical trials for Fragile X syndrome are nearing completion. Enrollment for the adult trial (EXPERIENCE-301) is now closed, while the adolescent trial (EXPERIENCE-204) is in its final phase. Learn more about the study, FRAXA’s role, and the open-label extension.
Read moreFRAXA-Funded Research Explores ISRIB as a Potential Treatment for Fragile X

ISRIB for Fragile X syndrome is being studied as a potential treatment to restore brain function and social behavior. Researchers investigate its effects.
Read moreHelp Rowan Thrive: Support Fragile X Research

Join the Gale family in supporting Fragile X research through FRAXA. Help Rowan and others thrive by funding life-changing treatments and advancing curative therapies.
Read moreSupport the Stevenson Family Campaign

The march of time renews the commitment we made to a special needs community 25 years ago. We vowed to dream big and never give up until there were effective treatments available and eventually a cure for Fragile X syndrome, the most commonly inherited cause of intellectual disabilities and autism.
Read moreMarvel Biosciences Partners with FRAXA to Test MB204 for Fragile X Syndrome

Discover how Marvel Biosciences and FRAXA Research Foundation are collaborating to test MB204, a promising new treatment for Fragile X syndrome, building on groundbreaking adenosine receptor research.
Read moreTo Interrogate the Developmental Timing for Treating Fragile X Syndrome
Are there critical periods in Fragile X syndrome? Will treatment work in adults as well as in children? This team aims to answer these questions.
Read moreBK Channel Openers: A New Drug for Fragile X Is Ready for Clinical Trials
Discover the promising new BK channel opener, SPG601, now entering clinical trials for Fragile X syndrome. Learn about its potential to restore synaptic function and address core symptoms.
Read moreFragile X Clinical Trial of New PDE4D Inhibitor from Tetra

With a $200,043 grant from FRAXA Research Foundation, Dr. Elizabeth Berry-Kravis completed a successful Phase 2 clinical trial of a PDE4 inhibitor for adult men with Fragile X syndrome. This trial treated 30 males, 18-45 years of age with a new PDE4D allosteric inhibitor from Tetra Discovery Partners using a crossover design, so that everyone got active drug for part of the time and placebo for part of the time.
Read moreContribution of Microglia to the Therapeutic Effects of Metformin and Adiponectin in Fragile X Syndrome

The research team of Brian Christie, PhD and Marie-Eve Tremblay is developing ways to balance hormones, including drugs like metformin and changes in diet, which could not only reduce hunger and obesity, but ultimately also improve learning and behavior in Fragile X syndrome.
Read moreBrain Organoids, Moving Fragile X Research Forward

There are many ways research produces discoveries, and all of them include a process of steps that build on each other. When an exciting new avenue appeared with potential for Fragile X syndrome, FRAXA stepped up to fund it. We now see the results of this grant and are excited to share them with you. The importance of different types of models have been shared and discussed over many years. We are now adding a “brain organoid” model to this group, and the potential behind it is really exciting.
Read morePromising Results of Preclinical Study of ANAVEX®2-73

We are excited to share that Anavex Life Sciences announced today that preclinical data of the ANAVEX®2-73 (blarcamesine) study in Fragile X syndrome were published in the peer-reviewed journal, Scientific Reports.
Read moreSynaptogenix Announced Intention to Launch a Fragile X Clinical Trial with Bryostatin

One of the most exciting kinds of work that FRAXA does is following the journey of an experimental new treatment until it is ready for trials in people with Fragile X. From an initial idea, through the development process, to clinical trials, FRAXA helps out all along the way. From an initial idea, through the development process, to clinical trials, FRAXA helps out all along the way. The recent announcement by Synaptogenix is a great example of how FRAXA funding and use of FRAXA-DVI can accelerate research on Fragile X.
Read moreCharacterization of Microglia Transcriptional Profile in Fmr1 Knockout Mice Model

With this grant, the team will identify the pathways responsible for this excessive activation and attempt to reverse the excess. If they can correct this using drugs, they will be able to identify a new potential treatment for Fragile X syndrome solving one more piece of the Fragile X brain puzzle.
Read moreScreening Combinatorial Pharmacological Therapies for Fragile X Syndrome
FRAXA Research Foundation has awarded a $90,000 research grant to Stanford University principal investigators Dr. Philippe Jacques Mourrain and Dr. Gordon Wang, along with postdoctoral fellow, Dr. Rochelle Coulson. They are evaluating additive effects of combinatorial drug treatments to correct a broad spectrum of deficits observed in Fragile X syndrome.
Read moreBryostatin-1 in Long-term Use Seen to Arrest Fragile X Symptoms in Mouse Model
Long-term, but not short-term, treatment with bryostatin-1 — Neurotrope’s lead investigational therapy — arrested such behavioral and cognitive symptoms as hyperactivity, difficulties with daily life activities, and learning and memory deficits in a mouse model of Fragile X syndrome.
Read morePositive Results Reported in Phase II Fragile X Clinical Trial of PDE4D Inhibitor Zatolmilast from Tetra Therapeutics

Today, Tetra Therapeutics announces the first unequivocally positive phase 2 clinical trial in Fragile X syndrome, press release below. The results do not depend on carving out a subset of patients or post hoc analysis.
Read moreScientists Find a New Way to Reverse Symptoms of Fragile X
FRAXA Investigator and MIT Professor Mark Bear and his colleagues have identified a valuable new target for Fragile X therapeutics: GSK3 alpha. Several FRAXA research teams previously identified GSK3 beta as a treatment target for Fragile X. The catch is that, so far, GSK3 beta inhibitors have proven too toxic for regular use. Dr. Bear’s new discovery opens up the possibility of developing more selective compounds with less toxicity and fewer side effects. Interestingly, lithium inhibits both GSK3 versions – alpha and beta.
Read moreCompanies Move to Advance Potential Cognitive Treatment for Fragile X

Tetra Therapeutics and Shionogi announced plans to expand their partnership supporting BPN14770, a treatment candidate for disorders marked by cognitive and memory deficits, including Fragile X syndrome and Alzheimer’s disease. The agreement builds on an earlier collaboration between the two companies, and aims to further accelerate BPN14770’s development and potential marketing. It is currently in clinical testing in both Fragile X and Alzheimer’s patients.
Read moreKetogenic Diet Eases Symptoms in Fragile X Male Mice

The Westmark laboratory continues to study sleep and rest-activity cycles in Fragile X mice as a potential outcome measure that correlates between preclinical and clinical research. The analysis of sleep EEG in the mice has proven more labor intensive than they anticipated, but the team is collaborating with Dr. Rama Maganti’s laboratory at UW-Madison on the development of computer scrips to speed up the analysis.
Read moreFRAXA Biotech Games, It Can Only Happen in an Open Community

The FRAXA Biotech Games exploded onto Cambridge Crossing with a capacity crowd. What was immediately obvious was the genuine camaraderie and mutual support of the biotech community and its many vendors to help raise awareness of and funds for research on Fragile X, the most common inherited cause of autism and intellectual disabilities.
Read moreTetra Announces $40M to Advance BPN14770 for FXS and Alzheimer’s Disease

Tetra Discovery Partners has signed a multi-part deal that could bring it up to $160 million, plus royalties, from Shionogi & Co, Ltd, a Japanese major research-driven pharmaceutical company. Tetra currently is conducting an investigational Phase 2 study of BPN14770 in adults with Fragile X Syndrome, an indication for which BPN14770 has received Orphan Drug Designation from the US Food and Drug Administration. This clinical trial was made possible by early work with the FRAXA-DVI and over $200,000 from FRAXA.
Read moreMetformin and Aberrant Insulin Signaling in a Fragile X Mouse Model

This 2017-2018 grant of $90,000 is funded jointly by FRAXA and the Fragile X Research Foundation of Canada for the first year. A previous FRAXA grant to the Sonenberg lab has led to great interest in the available drug, metformin, as a potential treatment for Fragile X syndrome. FRAXA is currently organizing clinical trials of metformin.
Read moreMega Green Tea Extract to Treat Fragile X?

Green tea is thought to have many benefits, particularly in cognitive function. In 2012-14, FRAXA Research Foundation funded a clinical trial to assess the effects of EGCG (green tea extract) on cognitive function in adults with FXS. Drs. Rafael de la Torre and Mara Dierssen Sotos, principal researchers in Barcelona, Spain, reported memory, attention, and mental flexibility improvements.
Read moreTetra Discovery Partners Initiates Phase 2 Trial of BPN14770 in Fragile X Syndrome

This 2-Period Crossover Study of BPN14770 is accepting adults males with Fragile X syndrome at Rush University Medical Center in Chicago. Principal Investigator of the study is Elizabeth Berry-Kravis, MD, PhD.
A selective inhibitor of the phosphodiesterase type-4D (PDE4D), BPN14770 has shown the ability to improve the quality of connections between neurons and to improve multiple behavioral outcomes in the Fragile X mouse model.

