Explore groundbreaking research on the potential of Cannabidiol (CBD) in modulating the endocannabinoid system for Fragile X syndrome therapy. Discover how CBD could change the natural course of Fragile X.
Read moreNovartis
FX-Learn Clinical Trial for Children with Fragile X

Thirteen centers across the US enrolled children with Fragile X in a large-scale clinical trial of Novartis AFQ056. Dr. Elizabeth Berry-Kravis and colleagues aim to show that this targeted treatment — an mGluR5 blocker for Fragile X which failed in previous adult human trials — can be better evaluated by studying effects on learning in young children.
Read moreCorrecting the Brain’s Emotional Memory Center
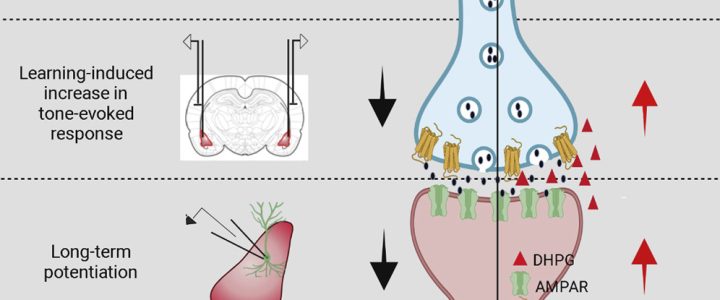
Cell Reports has published the results of the study “Correction of Amygdalar Dysfunction in Rat Model of Fragile X Syndrome” by FRAXA Investigator Dr. Sumatra Chattarji and his team at the National Centre for Biological Sciences in Bangalore, India. The researchers investigated the synaptic basis of deficient conditioned fear and its reversal in Fragile X syndrome rats.
Read moreDrug Tolerance in MGluR5 Clinical Trials – Dr Patrick McCamphill 1:1 with FRAXA

We have long suspected that the clinical trials of mGluR5 blockers from Novartis and Roche failed because the drug triggered tolerance, losing effect over time. With a $90,000 grant from FRAXA, Dr. Patrick McCamphill, a Postdoctoral Fellow in the MIT lab of Dr. Mark Bear, is investigating. He does indeed find tolerance, and now he is looking for ways to overcome it.
Read moreParkinson’s Therapy May Hold Promise for Fragile X

A study funded by FRAXA in Italy has encouraging results for people with Fragile X: drugs that block adenosine receptors (A2A) reversed signs of Fragile X in a mouse model.
“One of the most intriguing things about this study is that it points to an entire drug class (not just the one drug used) as potentially therapeutic for Fragile X. Many available compounds block A2A receptors, and we know they are safe and effective.
Read morefNIRS to Measure Treatment Response in Young Children with Fragile X

FRAXA Research Foundation has awarded a $90,000 research grant to Dr. Craig Erickson and Dr. Elizabeth Smith at Cincinnati Children’s Hospital to test functional near-infrared spectroscopy (fNIRS), in children who have Fragile X syndrome. fNIRS is safe, non-invasive, and easily-tolerated. It uses light sources and sensors on the scalp to build a heat map of the brain in action.
Read morePharmacological Tolerance in the Treatment of Fragile X Syndrome

With a $90,000 grant from FRAXA Research Foundation over 2018-2019, Dr. Patrick McCamphill, postdoctoral fellow in Dr. Mark Bear’s lab at Massachusetts Institute of Technology (MIT), is investigating drug tolerance to mGluR5 antagonists, arbaclofen, and other potential Fragile X treatments. He is also exploring ways to overcome it.
Read moreMechanisms of Tolerance to Chronic mGluR5 Inhibition

Over the past few years, both Novartis and Roche sponsored large-scale clinical trials of metabotropic glutamate receptor 5 (mGlu5) negative allosteric modulators (NAMs) to treat Fragile X syndrome (FXS). With a $90,000 grant from FRAXA Research Foundation in 2015-2017, Dr. Mark Bear’s team will explore if mGlu5 NAMs dosed chronically causes tolerance, and if so, how it develops and to probe new avenues to prevent or circumvent it.
Read moreNew Fragile X Clinical Trial for Children Launching in June 2017

Rush University Medical Center Professor Elizabeth M. Berry-Kravis, MD, PhD, has launched and is recruiting participants for a large-scale clinical trial to study effects of AFQ056, an mGluR5 blocker, on learning in young children.
Read moreWhy Did Fragile X Clinical Trials of mGluR Antagonists Fail?

by Michael Tranfaglia, MD. In my opinion, the Fragile X clinical trials of AFQ056 sponsored by Novartis failed because of a dose range that was inadequate for Fragile X, and because of the unexpected development of tolerance.
Read moreFragile X Clinical Trial: Novartis Trial Results Are In, and They’re Not Pretty
This year’s Gordon Conference just finished, and Novartis presented their results for the first time (though advisors and advocates had been given a private peak months ago.) To say that the trial results for AFQ056 were disappointing would be the understatement of the century!
Read moreNovartis Discontinues Development of mavoglurant (AFQ056) for Fragile X Syndrome
Novartis has announced that the company will be discontinuing its development program in Fragile X for its lead mGluR5 antagonist, mavoglurant (AFQ056), following negative results in a large international clinical trial in adults (reported in the Fall of 2013) and most recently, in a trial in adolescents. In both placebo-controlled trials, patients taking mavoglurant did not show improvement over placebo in any outcome measures. Novartis has also announced that the current open-label extension phase of the trial will be closed, but patients will be allowed to continue on the medication until their next scheduled clinic visit, or August 29, whichever comes first.
Read moreSocial Behavior as an Outcome Measure for Fragile X Clinical Trials

One of the features of the Fragile X mouse model which is relevant to the human Fragile X syndrome (and autism) is social behavior. Several tests show consistent social behavioral abnormalities in the Fragile X mouse model. With a $140,000 grant from FRAXA Research Foundation in 2012-2013, Dr. Willemsen at Erasmus University used social behavior tests to measure the effectiveness of several drug strategies.
Read moreClinical Trials FAQ ← Frequently Asked Questions
Question: How Do Families Decide Which Trial is Best for Them? Answer: Each of the trials has different requirements for joining, so many – if not most – people will only be eligible for one trial after screening. The best way to approach this is to call the clinic contact closest to your area and discuss this with him/her. Age, weight, current medications, behavior, and IQ are all factors.
Read more
