Updated 2025-03-21. Enrollment for the adult trial (EXPERIENCE-301) has now closed, and the adolescent trial (EXPERIENCE-204) is in its final phase. Please see below for Shionogi's official update.
Enrollment Update: Shionogi’s EXPERIENCE Trials Near Completion
We have an update on the progress of Shionogi’s EXPERIENCE clinical trials (also known as BPN14770-CNS-204, BPN14770-CNS-301, BPN14770-CNS-302 and the Tetra studies): As a result of an unprecedented surge of interest by this incredible community, enrollment for the adult study in Fragile X syndrome (EXPERIENCE-301) is nearly at capacity and screening will close today. Clinical trial sites are working with Shionogi to accommodate previously scheduled appointments for potential new study participants. Notably, clinical trial sites are no longer able to accommodate new appointments as the clinical trial cannot recruit far beyond the planned 150 individuals without putting the protocol integrity of the trial at risk and exhausting the total clinical trial drug supply.
The adolescent study in Fragile X syndrome (EXPERIENCE-204) is in its final phase of enrollment. All scheduled screening appointments will proceed as planned; however, no additional screening appointments are available. On behalf of our team and our partners at Shionogi, we are grateful for the support of the community in both raising awareness of and participating in these studies.
Please note the open-label study (EXPERIENCE-302) is ongoing for individuals who have completed EXPERIENCE-301 or 204. We look forward to sharing the results from these studies.

Background on the EXPERIENCE Trials
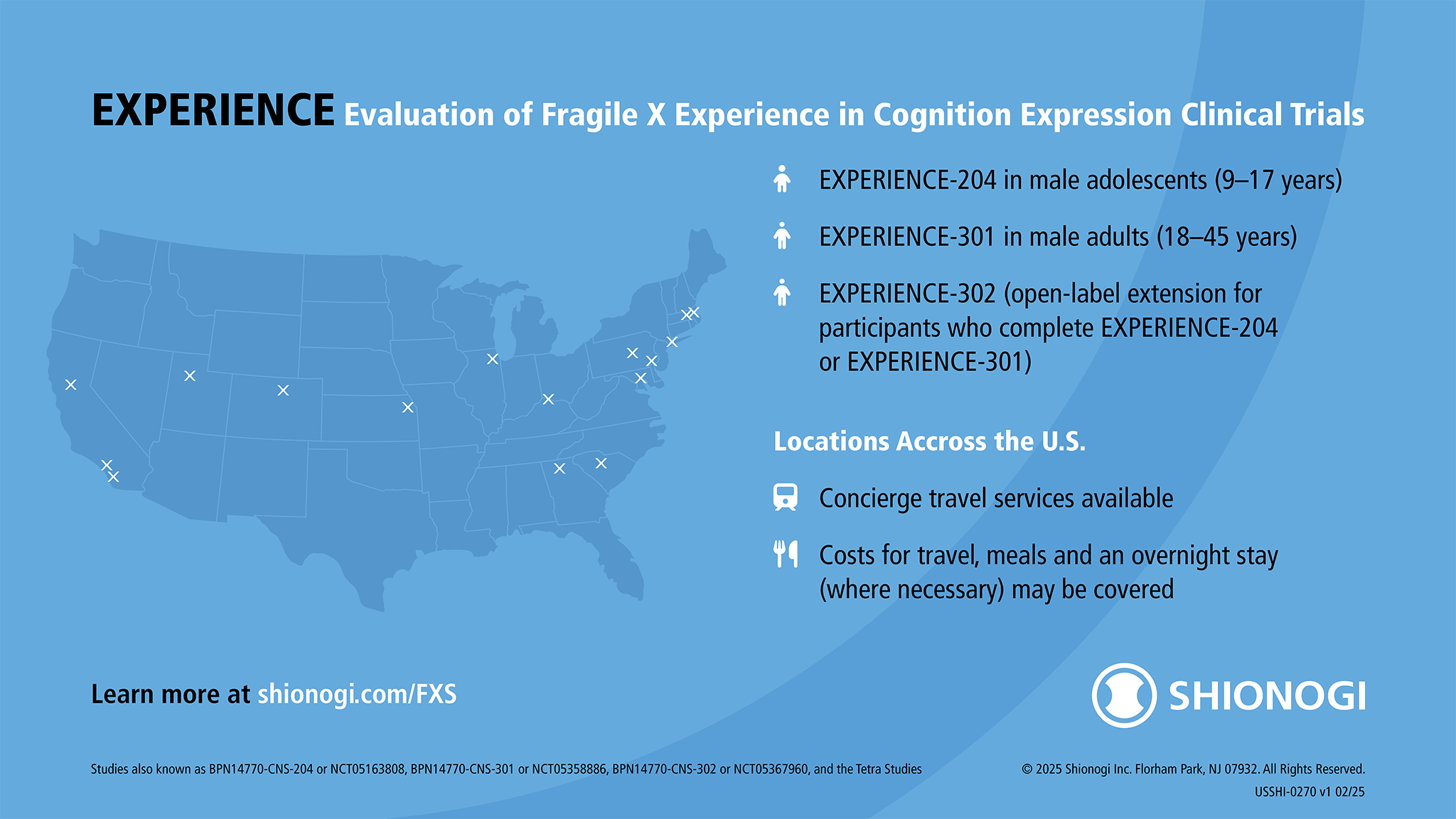
The EXPERIENCE clinical studies, Shionogi’s ongoing Phase 2b/3 studies for an investigational drug for Fragile X syndrome, previously recruited qualified male participants aged 9–45 with a confirmed FMR1 gene mutation (≥ 200 CGG repetitions) at sites across the U.S.
You may have heard about EXPERIENCE (Evaluation of Fragile X Experience in Cognition Expression) as the Tetra studies or the studies of BPN14770. These studies are now being managed by Shionogi, who acquired Tetra Therapeutics in 2020.
Travel for study participants and their caregivers may be covered and can include transportation, lodging, and meal reimbursement (limitations may apply).
Find out about EXPERIENCE at www.shionogi.com/FXS. You can also contact Shionogi directly at medinfo@shionogi.com or 1 (800) 849-9707.
FRAXA's Role in Supporting This Research
These trials mark a major milestone for community-based drug development. FRAXA-funded research pointed the way to phosphodiesterase inhibitors to treat Fragile X many years ago. However, finding a good phosphodiesterase inhibitor was a challenge because there was so much interest in the pharmaceutical world in developing these drugs for Alzheimer's disease and other blockbuster indications.
FRAXA then partnered with Tetra Therapeutics to test their advanced PDE 4D inhibitor in Fragile X mice, with remarkable results.
The results in the mice justified clinical trials, so FRAXA and Tetra organized and co-funded the first Fragile X trials of this investigational drug. The outcome was a genuine breakthrough for the field: statistically significant improvement in Fragile X subjects on a wide range of outcome measures. Behavior improved, quality of life improved, electrophysiology improved, and most importantly of all, cognition improved markedly. There are no drugs currently approved to treat intellectual disability.
In 2020, Tetra Therapeutics became a wholly owned subsidiary of Shionogi & Co., Ltd., enabling the continuation and expansion of this research under Shionogi’s leadership.
If the new trials are successful, our community could see the first approved treatment for Fragile X syndrome on the market.
Enrollment Information
Participation in this clinical trial includes reimbursement for expenses. This can include prepaid travel arrangements (transportation and hotel accommodation) and meal expenses, subject to certain restrictions.
UC Davis Health System
Davis, CA
Accepting males ages 9-45
Abigail Higareda Borbe
amoradelhigareda@ucdavis.edu
(916) 703-0281
Amnova Clinical Research
Irvine, CA
Accepting males ages 9-45
Erika Jacobo
erika.jacobo@amnovaresearch.com
(323) 348-0956
CHOC Thompson Autism Center
Orange, CA
Accepting males ages 9-45
Ulises Bustos
Ulises.Bustos@choc.org
(714) 288-7678
Children's Hospital Colorado
Aurora, CO
Accepting males ages 9-45
Erika Peterson
erika.peterson@childrenscolorado.org
(720) 777-8590
University of Miami
Miami, FL
Accepting males ages 9-45
Daniela Martinez
dmartinez2@med.miami.edu
(305) 243-3110
Emory University
Atlanta, GA
Accepting males ages 9-45
Jean Luan McColl
jean.luan@emory.edu
(404) 778-8619
Rush University
Chicago, IL
Accepting males ages 9-45
Loren Escot (Co-Coordinator)
Loren_Escot@rush.edu
(312) 942-2164
Abigail Ayemoba (Co-Coordinator)
abigail_ayemoba@rush.edu
(312) 942-2815
University of Kansas Medical Center
Kansas City, KS
Accepting males ages 9-45
Danielle Adam (Lead Coordinator)
dadam@kumc.edu
(913) 588-0494
Jasli Bonilla (Coordinator)
jbonilla@kumc.edu
(913) 945-9790
Boston Children's Hospital
Boston, MA
Accepting males ages 9-45
Michelle Coughlin
michelle.coughlin@childrens.harvard.edu
(617) 355-7025
UMass Chan Medical School
Worcester, MA
Accepting males ages 9-45
Lindsey Cincotta
lindsey.cincotta@umassmed.edu
(508) 856-7892
Kennedy Krieger Institute
Baltimore, MD
Accepting males ages 9-45
Maria Halaguena
halaguena@kennedykrieger.org
(443) 923-3826
Icahn School of Medicine
New York, NY
Accepting males ages 9-45
Venus Fan
venus.fan@mssm.edu
(929) 989-7016
Cincinnati Children's Hospital Medical Center
Cincinnati, OH
Accepting males ages 9-45
Elizabeth Blank
elizabeth.blank@cchmc.org
(513) 517-1550
Suburban Research Associates
Media, PA
Accepting males ages 9-45
Scott Comegys (Recruitment Specialist)
volunteer@atlas-clinical.com
610-891-7200
Clinic for Special Children
Strasburg, PA
Accepting males ages 9-45
Joelle Williamson
jwilliamson@
(717) 687-9407
Greenwood Genetic Center
Greenwood, SC
Accepting males ages 9-18
Nicole Johnston
njohnston@ggc.org
(864) 678-6912
University of Utah/Primary Children's Hospital
Salt Lake City, UT
Accepting males ages 9-18
Hina Yazdani
hina.yazdani@hsc.utah.edu
(801) 631-1067
Post Revisions
- 2025/02 - Updated with additional recruiting sites and EXPERIENCE branding.
- 2025/01 - Updated to reflect additional recruiting sites and the transition of Tetra Therapeutics to Shionogi.
- 2024/02 - Updated to include new age range, protocol amendments and additional recruiting sites.
- 2023/10 - Updated to include open label extension trial.
- 2023/06 - Updated with additional recruiting sites.
- 2022/10 - Updated as clinical trial now recruiting at first location.
- 2022/07 - Original post published.